Talk:Ethylene oxide
A fact from Ethylene oxide appeared on Wikipedia's Main Page in the Did you know column on 3 July 2010 (check views). The text of the entry was as follows:
|
| This article is rated B-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| This article contains a translation of Окись этилена from ru.wikipedia. |
Untitled
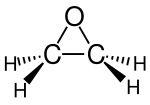
[edit]I don't see any oxygen in the structure diagram. Am I missing something? The image is
Image:Ethylene oxide.png
which is identical to
Image:Ethene.png
Hmmm... I clicked on Reload and it appeared...
Duplicate page
[edit]I notice that there is another (and IMHO much inferior) page on this compound at epoxyethane. I would like to turn that page into a simple redirect to this page. Any comments? Walkerma 06:10, 19 Mar 2005 (UTC)
IUPAC name
[edit]The article states that the IUPAC name of ethylene oxide is "1,2-epoxyethane". I don't think the numbers are needed in this case, since there is no ambiguity in the name "epoxyethane", and according to the naming guidelines, position numbers should not be used when not necessary. 193.217.163.93 14:26, 3 October 2006 (UTC)
New External Link
[edit]I added a new external link to http://www.eosa.org on the article page. This site deals with the safe use of Ethylene Oxide.
--Scot1967 (talk) 16:08, 5 January 2009 (UTC)
Old chembox
[edit]I'm moving the old chemical infobox here, as I've replaced it with a chembox new.
Ben 18:14, 9 September 2007 (UTC)
|
General |
|
|---|---|
| Name | Epoxyethane |
| Chemical formula | C2H4O |
| Formula weight | 44.05 u |
| Synonyms | Ethylene oxide, dimethylene oxide, oxirane, oxacyclopropane |
| SMILES | C1CO1 |
| CAS number | 75-21-8 |
| UN number | 1040 |
|
Phase behavior |
|
| Melting point | 161 K (-112.1 °C) |
| Boiling point | 283.5 K (10.4 °C) |
| Thermal decomposition | ? K (? °C) |
| Triple point | 160.6 K (-112.4 °C)
0.0078 kPa |
| Critical point | 468.9 K (195.9 °C)
72.3 kPa |
| ΔfusH | 5.17 kJ/mol |
| ΔfusS | 32.2 J/(mol·K) |
| ΔvapH | 25.5 kJ/mol |
| Solubility | Miscible with water. |
|
Liquid properties |
|
| ΔfH0liquid | -96 kJ/mol |
| S0liquid | 149.45 J/{mol·K) |
| Cp | 86.9 J/(mol·K) |
| Density | 0.899 ×103 kg/m3 |
|
Gas properties |
|
| ΔfH0gas | -52.6 kJ/mol |
| S0gas | 243 J/(mol·K) |
| Cp | 47 J/(mol·K) |
|
Safety | |
| Acute effects | Lung irritation, convulsions. |
| Chronic effects | CNS damage Potential carcinogen |
| Flash point | -55 °C |
| Autoignition temperature | 429 °C |
| Explosive limits | 3 to 100% |
|
More info | |
| Properties | NIST WebBook |
| MSDS | Hazardous Chemical Database |
|
SI units were used where possible. Unless otherwise stated, standard conditions were used. | |
Epoxyethane
[edit]Is there a reason I have missed why this page makes no mention of the name epoxyethane whatsoever? I was under the impression that is a fairly common name for this compound. I-hunter (talk) 20:42, 21 February 2009 (UTC)
- It's listed as the IUPAC name. If you click Show you'll see it. If we can confirm that epoxyethane is indeed the correct IUPAC name, then we should perhaps mention it in the lead. Thanks, Walkerma (talk) 21:12, 21 February 2009 (UTC)
Use in medical sterilization
[edit]Ethylene oxide is commonly used in medical sterilization, it would be nice if a little info on its use and process for this type sterilization was included. —Preceding unsigned comment added by 130.216.208.11 (talk) 23:49, 1 July 2010 (UTC)
- There is some "little info" on that in the lead and "applications", "other uses". Materialscientist (talk) 23:52, 1 July 2010 (UTC)
It says "As a toxic gas that leaves residue on items it contacts". Should that be "leaves NO residue"? Thirteenangrymen (talk) 22:29, 16 March 2020 (UTC)
History of Synthesis
[edit]"The first synthesis method had long remained the only, despite numerous attempts of scientists, including Wurtz himself, to produce ethylene oxide directly from ethyl". Should "ethyl" not read "ethanol" or "ethylene/ethene"? Moletrouser (talk) 05:27, 9 August 2010 (UTC)
"China Petrochemical Corporation (~1000 tonnes in 2006[68])"
[edit]Taken from this version of the article. There's something very likely wrong with this. The number is three orders of magnitude below the other productions mentioned nearby. The source cited requires registration. 80.250.81.35 (talk) 08:34, 11 December 2012 (UTC)
- 1 million, not 1000, probably my typo, fixed, thanks. Materialscientist (talk) 08:46, 11 December 2012 (UTC)
Structure of ethylene oxide: history
[edit]The article states: "The heterocyclic triangular structure of ethylene oxide was not proposed until 1893." However, I suspect that the triangular structure of ethylene oxide was proposed (and in use in the literature) earlier than 1893.
In the journal Nature (April 6, 1893, vol. 47, p. 551), a report states: "The author then proceeds to discuss the orthodox formulae for trimethylene [i.e., cyclopropane], ethylene oxide, and diazoimide [i.e., hydrazoic acid] …". There follows an illustration showing the hypothetical triangular structure of each substance. "The author" (W. H. Perkin) calls these structures "orthodox", which suggests that they've been used widely for some time; i.e., prior to 1893. Ethylene oxide was depicted as having a triangular molecular structure in:
- 1890: Henry E. Roscoe and Carl Schorlemmer, ed.s A Treatise on Chemistry (New York: D. Appleton and Co., 1890), vol. 3 (The chemistry of the hydrocarbons …), part 2, page 35.
- 1889: Henry Watts et al., ed.s, Watts' Dictionary of Chemistry (London, England: Longmans, Green, and Co.: 1889), vol. 2, page 490.
- 1883: Friedrich K. Beilstein, Handbuch der organischen Chemie (Hamburg, Germany: Leopold Voss, 1883), vol. 1, page 392.
- 1876: Eugen F. von Gorup-Besanez, ed., Lehrbuch der organischen Chemie für den Unterricht auf Universitäten, … (Braunschweig, Germany: Friedrich Vieweg und Sohn, 1876), vol. 2, page 253.
Cwkmail (talk) 18:36, 21 March 2013 (UTC)
Cleansing of typography
[edit]A followup of user talk: Materialscientist #<math> pollution.
I think either “⋯” or “ · · · ” will be possible replacements for a moronic “•••”. Though, “•” for a free radical (i.e. an unpaired electron) is nice, isn’t it? Or, possibly, it should be superscripted, such as in •OH? Incnis Mrsi (talk) 14:42, 29 June 2013 (UTC)
Thermodynamic values
[edit]I received the following message from "DVWynn", who noted a difference between 2 sources for the compound's heat of formation--
In The Chemistry of Heterocyclic Compounds, Small Ring Heterocycles edited by Alfred Hassner, page 7-8, the heat of formation [of etylene oxide] is given as 117.2 kJ/mol, [which is] derived from heat of combustion 114.4 kJ/mol, and importantly, the ring-strain is quoted as 54.4 kJ/mol. These are at odds with the values on the page by a HUGE amount. [The ethylene oxide page lists the compound's heat of formation as: Std enthalpy of formation ΔfHo298 = −52.6 kJ mol−1 .] I presume 'angular stress' means ring-strain but maybe my terminology is out of date? Since its main use is in thermobaric weapons, I presume that it packs a lot of energy in a small (by spacial volume) 'package'. Note that heptanitrocubane is preferred over octanitrocubane because its concentration of energy (due to crystal structure) is higher.
Cwkmail (talk) 16:54, 9 July 2013 (UTC)
I did some very quick checking and confirmed that Hassner's book The Chemistry of Heterocyclic Compounds … does indeed list the heat of formation of ethylene oxide as 117.2 kJ/ mol. (See: Heterocyclic Compounds ... by A. Hassner ) However, a couple of other sources may explain the difference in the figures for the compound's heat of formation. The Wikipedia article lists the heat of formation for the compound in its GAS state, whereas the heat of formation is higher for the compound in its LIQUID state. Hassner may have listed the heat of formation for the compound in its LIQUID state.
For example, on page 12 of this pdf file -- American Chemistry.com -- the heat of formation of the GASEOUS form is listed as
-1,194.8 kJ/kg = -52.6 kJ/mol ;
however, the heat of formation of the LIQUID form is listed as
-1,766.5 kJ/kg = -77.8 kJ/mol .
The National Institute of Standards and Technology lists the compound's heat of formation in the GASEOUS state as
-52.6 kJ/mol
However, in the "Comment" section, the heat of formation in the LIQUID state is listed as
-95.7 kJ/mol
So it seems likely that the difference in the heats of formation are the result of using different states (liquid vs. gas) as references for the measurements. However, in order to confirm this, it would be necessary to view Hassner's sources (which he does list). Cwkmail (talk) 17:53, 9 July 2013 (UTC)
Suggestions
[edit]About Ehtylene Oxide producers: in case anyone has the time or care to add this to the article, LyondellBasell also produces this product in Texas, USA according to their website. Reference: Http://www.lyondellbasell.com/Aboutus/WorldWideLocations/NorthAmerica/USA/Texas/Channelview/ — Preceding unsigned comment added by 98.194.50.35 (talk) 15:22, 8 April 2014 (UTC)
NFPA rating is wrong
[edit]According to the diagram it is a 3-4-3 rating (NFPA 704) but when I looked at the source (http://web.archive.org/web/20090804080033/http://www.sonoma-county.org/des/pdf/fire/bulletins/info_bulletin_nfpa_marking2009_04n.pdf) it is actually a 2-4-3. — Preceding unsigned comment added by 2001:610:1908:F000:D82E:D0AA:9CF2:39D3 (talk) 12:02, 27 May 2014 (UTC)
External links modified
[edit]Hello fellow Wikipedians,
I have just added archive links to 10 external links on Ethylene oxide. Please take a moment to review my edit. You may add {{cbignore}} after the link to keep me from modifying it, if I keep adding bad data, but formatting bugs should be reported instead. Alternatively, you can add {{nobots|deny=InternetArchiveBot}} to keep me off the page altogether, but should be used as a last resort. I made the following changes:
- Attempted to fix sourcing for http://www.scidesign.com/Business/EO%20-%20EG%20Process/EO_EG_Process.htm
- Attempted to fix sourcing for http://www.shell.com/home/content/chemicals/products_services/our_products/ethylene_oxide_glycols/ethylene_glycols/manufacturing_locations/geismar/
- Attempted to fix sourcing for http://www.shell.com/home/content/chemicals/products_services/our_products/ethylene_oxide_glycols/ethylene_glycols/manufacturing_locations/moerdijk/
- Attempted to fix sourcing for http://www.ineosoxide.com/21-Ethylene_Oxide__EO_.htm
- Attempted to fix sourcing for http://www.shell.com/home/content/chemicals/products_services/our_products/ethylene_oxide_glycols/ethylene_glycols/product_overview/
- Attempted to fix sourcing for http://www.buss-ct.com/e/reaction_technology/alkoxylation.php?navid=36
- Attempted to fix sourcing for http://www.environmentwriter.org/resources/backissues/chemicals/ethylene_oxide.htm
- Attempted to fix sourcing for http://cal.vet.upenn.edu/projects/surgery/2220.htm
- Attempted to fix sourcing for http://www.safework.ru/ilo/ICSC/cards/view/?0155
- Attempted to fix sourcing for http://www.sonoma-county.org/des/pdf/fire/bulletins/info_bulletin_nfpa_marking2009_04n.pdf
When you have finished reviewing my changes, please set the checked parameter below to true or failed to let others know (documentation at {{Sourcecheck}}).
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—cyberbot IITalk to my owner:Online 13:45, 29 March 2016 (UTC)
Assessment comment
[edit]The comment(s) below were originally left at Talk:Ethylene oxide/Comments, and are posted here for posterity. Following several discussions in past years, these subpages are now deprecated. The comments may be irrelevant or outdated; if so, please feel free to remove this section.
| I find the upper flammability or explosive limit of 100% disturbing. If 100% is possible what makes ethylene oxide special? |
Last edited at 08:24, 1 October 2009 (UTC). Substituted at 14:44, 29 April 2016 (UTC)
Ethylene oxide is non-chiral
[edit]"In 2016, ethylene oxide became the first known chiral molecule detected in space.[1]"
The article references to propylene oxide.
- Removed, thanks. Materialscientist (talk) 10:48, 13 November 2016 (UTC)
References
- ^ McGuire, Brett A.; Carroll, P. Brandon; Loomis, Ryan A.; Finneran, Ian A.; Jewell, Philip R.; Remijan, Anthony J.; Blake, Geoffrey A. (2016-06-17). "Discovery of the interstellar chiral molecule propylene oxide (CH3CHCH2O)". Science. 352 (6292): 1449–1452. doi:10.1126/science.aae0328. ISSN 0036-8075.
Charge balance
[edit]Our article gives a reaction mechanism suggested by Kilty and Sachtler, but two of the reactions are not charge-balanced. I can't access the journal article. Can someone fix this? Eric Kvaalen (talk) 14:20, 24 July 2021 (UTC)
- I downloaded the paper. If you have any questions, feel free to ask. The authors draw a picture with Ag-O--O+. The terminal O attacks the C=C bond.--Smokefoot (talk) 21:57, 24 July 2021 (UTC)
Units of concentration and temperature
[edit]Units are all over the place. Sometimes degrees Celsius is converted in parens to Fahrenheit, sometimes not. As far as I can tell and just checking mentally, the conversions have been made accurately but I have not checked them all. I don't see the need for these conversions because the only common use for Fahrenheit is ambient temperature and cooking temperature in the countries which have not adopted Celsius, and they disrupt the flow of the piece. Concentration of EtO in air from the point of view of toxicity are given in ppm, mL/m3, imp fl oz/cu ft (!! yes really), mg/m3, gr/cu ft (note here gr = grains not grams - again it appears that the conversion to gr/cu ft has been done accurately but come on - do we really want grains in WP articles?). I propose that we use the reference's units - which of course vary - and convert all these to either ppm or mg/m3 a common lingua franca unit as necessary. This will be a lot of work and I do actually know what I am doing, but I am worried that it would be seen by some as OR: and extensive work to clarify the article to make it more useful would be reverted. Any thoughts? Cross Reference (talk) 01:12, 27 November 2022 (UTC)
- Wikipedia Did you know articles
- B-Class chemicals articles
- High-importance chemicals articles
- B-Class WPChem worklist articles
- B-Class Occupational Safety and Health articles
- High-importance Occupational Safety and Health articles
- WikiProject Occupational Safety and Health articles
- B-Class Environment articles
- Mid-importance Environment articles
- Pages translated from Russian Wikipedia