DEET
| |

| |
| Names | |
|---|---|
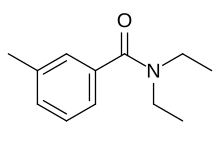
| Preferred IUPAC name
N,N-Diethyl-3-methylbenzamide | |
| Other names
N,N-Diethyl-m-toluamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.682 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H17NO | |
| Molar mass | 191.27 g/mol |
| Density | 0.998 g/mL |
| Melting point | −33 °C (−27 °F; 240 K) |
| Boiling point | 288 to 292 °C (550 to 558 °F; 561 to 565 K) |
| Pharmacology | |
| P03BX02 (WHO) QP53GX01 (WHO) | |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H302, H315, H319, H402 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
N,N-Diethyl-meta-toluamide, also called diethyltoluamide or DEET (/diːt/, from DET, the initials of di- + ethyl + toluamide),[1][2] is the oldest, one of the most effective and most common active ingredient in commercial insect repellents. It is a slightly yellow oil intended to be applied to the skin or to clothing and provides protection against mosquitoes, flies, ticks, fleas, chiggers, leeches, and many other biting insects.
Unlike icaridin, DEET emits an odor that many find unpleasant, leaves skin greasy, dissolves plastics and synthetic fabrics[3] and interacts negatively with sunscreen.[4][5]
Effectiveness
[edit]DEET and icaridin are the most effective insect repellents available.[6] DEET is effective against a variety of invertebrates, including ticks, flies, mosquitos, and some parasitic worms.[6]
A 2018 systematic review found no consistent performance difference between DEET and icaridin in field studies and concluded that they are equally preferred mosquito repellents, noting that 50% DEET offers longer protection but is not available in some countries.[7]
Concentrations
[edit]The concentration of DEET in products may range from less than 10% to nearly 100%, but concentrations greater than 50% do not increase the duration of protection.[8] Higher concentrations can be safely applied to clothing, although it may damage some types of synthetic fibers. In the United Kingdom, the publicly-funded healthcare system, the National Health Service (NHS), recommends that UK citizens should use a concentration of 50% when visiting areas of the world with malaria.[9] A lower concentration of 10% is recommended for infants and children.[10][11] Health Canada decided to limit DEET concentration to 30% in the country since 2002 due to an increased long-term risk observed with repeated applications.[12]
DEET is often sold and used in spray or lotion in concentrations up to 100%.[13] Consumer Reports found a correlation between DEET concentration and hours of protection against insect bites. 100% DEET was found to offer up to 12 hours of protection while several lower concentration DEET formulations (20–34%) offered 3–6 hours of protection.[citation needed] Other research has corroborated the effectiveness of DEET.[14] The Centers for Disease Control and Prevention (CDC) recommends 30–50% DEET to prevent the spread of pathogens carried by insects.[15]
A 2008 study found that higher concentrations of DEET have an improved ability to repel insects through fabric.[16]
Contraindications
[edit]DEET should not be used on children younger than 2 months of age.[17]
Adverse effects
[edit]When used as directed, products containing between 10% and 30% DEET have been found by the American Academy of Pediatrics to be safe to use on children as well as adults.[17]
As a precaution, manufacturers advise that DEET products should not be used under clothing or on damaged skin, and that preparations be washed off after they are no longer needed or between applications.[17] DEET can irritate the eyes and, unlike icaridin, it can cause breathing difficulty, headaches,[18] or, in rare cases, it may cause severe epidermal reactions.[17]
The authors of a 2002 study published in The New England Journal of Medicine wrote:[19]
... this repellent has been subjected to more scientific and toxicologic scrutiny than any other repellent substance. ... DEET has a remarkable safety profile after 40 years of use and nearly 8 billion human applications. Fewer than 50 cases of serious toxic effects have been documented in ... medical literature since 1960 ... Many of these cases of toxic effects involved long-term, heavy, frequent, or whole-body application of DEET. No correlation has been found between the concentration of DEET used and the risk of toxic effects. ... When applied with common sense, DEET-based repellents can be expected to provide a safe as well as a long-lasting repellent effect ... under circumstances in which it is crucial to be protected against arthropod bites that might transmit disease.
In the DEET Reregistration Eligibility Decision (RED) in 1998, the United States Environmental Protection Agency (EPA) reported 14 to 46 cases of potential DEET-associated seizures, including four deaths. The EPA states: "... it does appear that some cases are likely related to DEET toxicity," which may underreport the risk as physicians may fail to check for history of DEET use or fail to report cases of seizure subsequent to DEET use.[20]
In 1997, the Pesticide Information Project of Cooperative Extension Offices of Cornell University stated that "Everglades National Park employees having extensive DEET exposure were more likely to have insomnia, mood disturbances and impaired cognitive function than lesser exposed co-workers".[21]
Citing human health reasons, Health Canada barred the sale of insect repellents for human use that contained more than 30% DEET in a 2002 re-evaluation "based on a human health risk assessment that considered daily application of DEET over a prolonged period of time". The agency recommended that DEET-based products be used on children between the ages of 2 and 12 only if the concentration of DEET is 10% or less and that repellents be applied no more than 3 times a day, children under 2 should not receive more than 1 application of repellent in a day and DEET-based products of any concentration should not be used on infants under 6 months.[22][23]
A 2020 study performed by students within the University of Florida's College of Public Health and Health Professions analyzed data from the National Health and Nutrition Examination Survey and identified 1,205 participants who had "DEET metabolic levels recorded at or above detection limits". They analyzed biomarkers related to systemic inflammation, immune, liver, and kidney functions, and found no "evidence that DEET exposure has any impact on the biomarkers identified."[24]
Detection in body fluids
[edit]DEET may be measured in blood, plasma, or urine by gas or liquid chromatography-mass spectrometry to confirm a diagnosis of poisoning in hospitalized patients or to provide evidence in a medicolegal death investigation. Blood or plasma DEET concentrations are expected to be in a range of 0.3–3.0 mg/L during the first 8 hours after dermal application in persons using the chemical appropriately, >6 mg/L in intoxicated patients and >100 mg/L in victims of acute intentional oral overdose.[25][26]
Overdose
[edit]Applying DEET to the skin is safe if done as directed. Adverse reactions are very rare, about 1 in 100 million people. However, repeated use of DEET in very high concentrations can lead to toxic encephalopathy with severe neurological symptoms including seizures, tremors and slurred speech. The risk is higher for children since they have a greater surface area to body weight ratio.[27][28]
Interactions
[edit]Limited data indicates that combining insect repellents with DEET and sunscreen decreases the sun protection factor of the sunscreen by about a third.[4] Unlike icaridin, the combination also increases the absorption of both significantly.[5] When the two need to be used together, the repellent should be applied after the sunscreen has been absorbed, about 30 or more minutes later.[29]
When DEET is used in combination with insecticides for cockroaches it can strengthen the toxicity of carbamate, an acetylcholinesterase inhibitor. These 1996 findings indicate that DEET has neurological effects on insects in addition to known olfactory effects, and that its toxicity is strengthened in combination with other insecticides.[30]
Damage to materials
[edit]Unlike icaridin, DEET is an effective solvent[31] and may dissolve some watch crystals,[32] plastics, rayon, spandex, other synthetic fabrics, and painted or varnished surfaces including nail polish. It also may act as a plasticizer by remaining inside some formerly hard plastics, leaving them softened and more flexible. DEET is incompatible with rayon, acetate, or dynel clothing.[33]
Environmental impact
[edit]Though DEET is not expected to bioaccumulate, it has been found to have a slight toxicity for fresh-water fish such as rainbow trout[34] and tilapia,[35] and it also has been shown to be toxic for some species of freshwater zooplankton.[36] DEET has been detected at low concentrations in water bodies as a result of production and use, such as in the Mississippi River and its tributaries, where a 1991 study detected levels varying from 5 to 201 ng/L.[37]
A 1975 study analyzed the effects of DEET on communities of freshwater organisms native to Chinese waterways and found that DEET was moderately toxic to aquatic organisms compared to other commercial insect repellants. The most-at-risk organisms were algae colonies which often experienced "significant biomass decline and community composition shift[s]" when exposed to DEET at 500 ng/L.[38]
DEET is biodegraded by fungi into products less toxic to zooplankton.[36] It degrades well under aerobic conditions, but poorly and slowly under anaerobic conditions.[39]
Mechanism of action
[edit]DEET is thought to provide protection from mosquitos via two pathways, both by negatively impacting mosquito odorant receptors at a distance, and by negatively impacting mosquito chemoreceptors upon contact.[6] The exact mechanisms are still being researched, but the two most likely hypotheses are the "smell and avoid hypothesis" (that DEET has an unpleasant odor to insects), and the "bewilderment hypothesis" (that smelling DEET confuses insects).[6] An alternative hypothesis is that DEET "masks" humans by reducing the volatility of skin odorants that are attractive to insects.[40]
Synthesis
[edit]A slightly yellow liquid at room temperature, it can be prepared by converting m-toluic acid (3-methylbenzoic acid) to the corresponding acyl chloride using thionyl chloride (SOCl2), and then allowing that product to react with diethylamine:[41][42]
History
[edit]DEET was developed in 1944[43] by Samuel Gertler[43] of the United States Department of Agriculture for use by the United States Army,[44] following its experience of jungle warfare during World War II. It was originally tested as a pesticide on farm fields, and entered military use in 1946 and civilian use in 1957. It was used in Vietnam and Southeast Asia.[45]
In its original form, known as "bug juice", the application solution was composed of 75% DEET and 25% ethanol.[32] Later, a new version of the repellent was developed by the U.S. Army and the USDA. This formulation consisted of DEET and a mixture of polymers that extended its release and reduced its evaporation rate.[46] This extended-release application was registered by the Environmental Protection Agency in 1991.[32]
See also
[edit]- Beautyberry
- Citronella oil
- DDT, another insecticide used for disease vector control
- Ethyl butylacetylaminopropionate (IR3535)
- Icaridin
- Lemon eucalyptus
- Mosquito coil
- p-Menthane-3,8-diol (PMD)
- Permethrin, a pyrethroid insecticide that can be applied to clothing to help prevent bites
- SS220
- Anthranilate-based insect repellents
References
[edit]- ^ "DEET". Merriam-Webster. Retrieved 2023-06-04.
- ^ Plumlee KH (2004-01-01), Plumlee KH (ed.), "Chapter 21 - Insecticides and Molluscicides", Clinical Veterinary Toxicology, Saint Louis: Mosby, pp. 177–192, doi:10.1016/b0-32-301125-x/50024-8, ISBN 978-0-323-01125-9
- ^ "Picaridin vs DEET: Which Is the Best Insect Repellent?". Appalachian Mountain Club. 4 August 2023. Retrieved 7 August 2023.
- ^ a b "Mosquitoes, Ticks & Other Arthropods - CDC Yellow Book 2024". Centers for Disease Control and Prevention. Retrieved 2023-09-26.
- ^ a b Rodriguez J, Maibach HI (2016-01-02). "Percutaneous penetration and pharmacodynamics: Wash-in and wash-off of sunscreen and insect repellent". Journal of Dermatological Treatment. 27 (1): 11–18. doi:10.3109/09546634.2015.1050350. ISSN 0954-6634. PMID 26811157. S2CID 40319483.
- ^ a b c d DeGennaro M (2015). "The mysterious multi-modal repellency of DEET". Fly. 9 (1): 45–51. doi:10.1080/19336934.2015.1079360. ISSN 1933-6942. PMC 4594586. PMID 26252744.
- ^ Goodyer L, Schofield S (2018-05-01). "Mosquito repellents for the traveller: does picaridin provide longer protection than DEET?". Journal of Travel Medicine. 25 (Suppl_1): S10–S15. doi:10.1093/jtm/tay005. ISSN 1708-8305. PMID 29718433.
- ^ "Mosquitoes, Ticks & Other Arthropods - CDC Yellow Book 2024". Centers for Disease Control and Prevention. Retrieved 2023-08-07.
- ^ Team HT. "Mosquito Bite Avoidance - Fit for Travel". www.fitfortravel.nhs.uk. Retrieved 2023-08-07.
- ^ Canada H (2012-05-11). "Personal Insect repellents". Government of Canada. Retrieved 2023-08-07.
- ^ American Academy of Pediatrics, “Summer Safety Tips,” Dec 2, 2017 https://www.healthychildren.org/English/safety-prevention/at-play/Pages/Summer-Safety-Tips-Staying-Safe-Outdoors.aspx
- ^ "Health Canada to ban bug repellents with high concentrations of DEET". CBC. 24 April 2002. Retrieved 7 August 2023.
- ^ DEET in the Consumer Product Information Database
- ^ Williamson D (3 July 2002). "Independent study: DEET products superior for fending off mosquito bites" (Press release). University of North Carolina. Archived from the original on 30 June 2007. Retrieved 19 May 2007.
- ^ "Protection against Mosquitoes, Ticks, Fleas and Other Insects and Arthropods". Travelers' Health – Yellow Book. Centers for Disease Control and Prevention. 2009-02-05. Archived from the original on 2009-05-05. Retrieved 2009-02-05.
- ^ Ditzen M, Pellegrino M, Vosshall LB (2008). "Insect odorant receptors are molecular targets of the insect repellent DEET". Science. 319 (5871): 1838–42. Bibcode:2008Sci...319.1838D. doi:10.1126/science.1153121. PMID 18339904. S2CID 18499590.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d "Insect Repellent Use and Safety". West Nile Virus. Centers for Disease Control and Prevention. 2007-01-12. Archived from the original on 23 May 2013.
- ^ "Bug spray poisoning". U.S. National Library of Medicine. October 2015. Retrieved 2016-06-25.
- ^ Fradin MS, Day JF (July 2002). "Comparative efficacy of insect repellents against mosquito bites". The New England Journal of Medicine. 347 (1): 13–8. doi:10.1056/NEJMoa011699. PMID 12097535.
- ^ "Reregistration Eligibility Decision: DEET" (PDF). U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances. September 1998. pp. 39–40. Archived from the original (PDF) on October 21, 2012. Retrieved 2012-09-08.
- ^ "DEET". Pesticide Information Profile. EXTOXNET. October 1997. Archived from the original on 2020-11-19. Retrieved 2007-09-26.
- ^ "Insect Repellents". Healthy Living. Health Canada. August 2009. Archived from the original on 2010-04-11. Retrieved 2010-07-09.
- ^ "Re-evaluation Decision Document: Personal insect repellents containing DEET (N,N-diethyl-m-toluamide and related compounds)" (PDF). Consumer Product Safety. Health Canada. 2002-04-15. Archived from the original (PDF) on 4 March 2021. Retrieved 2010-07-09.
- ^ Haleem ZM, Yadav S, Cushion ML, Tanner RJ, Carek PJ, Mainous AG (2020). "Exposure to N,N-Diethyl-Meta-Toluamide Insect Repellent and Human Health Markers: Population Based Estimates from the National Health and Nutrition Examination Survey". Am J Trop Med Hyg. 103 (2): 812–814. doi:10.4269/ajtmh.20-0226. PMC 7410448. PMID 32458781.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tenenbein M (September 1987). "Severe toxic reactions and death following the ingestion of diethyltoluamide-containing insect repellents". JAMA. 258 (11): 1509–11. doi:10.1001/jama.258.11.1509. PMID 3625951.
- ^ Baselt RC (2014). Disposition of toxic drugs and chemicals in man, 10th edition. Seal Beach, Ca.: Biomedical Publications. p. 650. ISBN 978-0-9626523-9-4.
- ^ "EWG's 2018 Guide to Bug Repellents". Environmental Working Group. 17 July 2018. Archived from the original on 10 April 2021. Retrieved 9 August 2023.
- ^ Toxicological profile for DEET (N,N-Diethyl-meta-toluamide) (Technical report). Agency for Toxic Substances and Disease Registry. August 2017. pp. 74–78.
- ^ "How to choose the best bug repellent". Best Health. Reader's Digest Association, Inc. January 2000. Retrieved June 14, 2016.
- ^ Moss JI (October 1996). "Synergism of toxicity of N,N-diethyl-m-toluamide to German cockroaches (Orthoptera: Blattellidae) by hydrolytic enzyme inhibitors". Journal of Economic Entomology. 89 (5): 1151–5. doi:10.1093/jee/89.5.1151. PMID 17450648.
- ^ Petherick A (2008-03-13). "How DEET jams insects' smell sensors". Nature News. doi:10.1038/news.2008.672. Retrieved 2008-03-16.
- ^ a b c Kitchen LW, Lawrence KL, Coleman RE (June 2009). "The role of the United States military in the development of vector control products, including insect repellents, insecticides, and bed nets". Journal of Vector Ecology. 34 (1): 50–61. doi:10.1111/j.1948-7134.2009.00007.x. PMID 20836805.[permanent dead link]
- ^ "Diethyltoluamide". PubChem. National Center for Biotechnology Information. Retrieved 7 August 2023.
- ^ U.S. Environmental Protection Agency. 1980. Office of Pesticides and Toxic Substances. N,N-diethyl-m-toluamide (Deet) Pesticide Registration Standard. December 1980. 83 pp.
- ^ Mathai AT, Pillai KS, Deshmukh PB (1989). "Acute toxicity of deet to a freshwater fish, Tilapia mossambica : Effect on tissue glutathione levels". Journal of Environmental Biology. 10 (2): 87–91. Archived from the original on 2007-11-07.
- ^ a b Seo J, Lee YG, Kim SD, Cha CJ, Ahn JH, Hur HG (April 2005). "Biodegradation of the insecticide N,N-diethyl-m-toluamide by fungi: identification and toxicity of metabolites". Archives of Environmental Contamination and Toxicology. 48 (3): 323–8. Bibcode:2005ArECT..48..323S. doi:10.1007/s00244-004-0029-9. PMID 15750774. S2CID 31723995.
- ^ Zeiger E, Tice R, Brevard B (1999). "N,N-Diethyl-m-toluamide (DEET) [134-62-3] – Review of Toxicological Literature" (PDF). Archived from the original (PDF) on October 9, 2012.
- ^ Schmoldt A, Benthe HF, Haberland G (1975). "Digitoxin metabolism by rat liver microsomes". Biochem Pharmacol. 24 (17): 1639–41. doi:10.1016/0006-2952(75)90094-5. hdl:10033/333424. PMID 10.
- ^ Weeks JA, Guiney PD, Nikiforov AI (January 2012). "Assessment of the environmental fate and ecotoxicity of N,N-diethyl-m-toluamide (DEET)". Integrated Environmental Assessment and Management. 8 (1): 120–34. Bibcode:2012IEAM....8..120W. doi:10.1002/ieam.1246. PMID 22006575. S2CID 5370204.
- ^ Afify A, Betz JF, Riabinina O, Lahondère C, Potter CJ (November 2019). "Commonly Used Insect Repellents Hide Human Odors from Anopheles Mosquitoes". Current Biology. 29 (21): 3669–3680.e5. Bibcode:2019CBio...29E3669A. doi:10.1016/j.cub.2019.09.007. PMC 6832857. PMID 31630950.
- ^ Wang BJ (1974). "An interesting and successful organic experiment (CEC)". J. Chem. Educ. 51 (10): 631. Bibcode:1974JChEd..51..631W. doi:10.1021/ed051p631.2.
- ^ Pavia DL (2004). Introduction to organic laboratory techniques (Google Books excerpt). Cengage Learning. pp. 370–376. ISBN 978-0-534-40833-6.
- ^ a b US 2408389, Gertler S, "N,N-diethylbenzamide as an insect repellent", published 1946-10-01
- ^ Katz TM, Miller JH, Hebert AA (May 2008). "Insect repellents: historical perspectives and new developments". Journal of the American Academy of Dermatology. 58 (5): 865–71. doi:10.1016/j.jaad.2007.10.005. PMID 18272250. Retrieved 2015-08-16.
- ^ Committee on Gulf War and Health: Literature Review of Pesticides and Solvents (2003). Gulf War and Health: Volume 2. Insecticides and Solvents. Washington, D.C.: National Academies Press. doi:10.17226/10628. ISBN 978-0-309-11389-2.
- ^ Kitchen LW, Lawrence KL, Coleman RE (2009-07-10). "The role of the United States military in the development of vector control products, including insect repellents, insecticides, and bed nets". Journal of Vector Ecology. 34 (1): 50–61. doi:10.1111/j.1948-7134.2009.00007.x. PMID 20836805.
Further reading
[edit]- Fradin MS (June 1998). "Mosquitoes and mosquito repellents: a clinician's guide". Annals of Internal Medicine. 128 (11): 931–40. CiteSeerX 10.1.1.691.2193. doi:10.7326/0003-4819-128-11-199806010-00013. PMID 9634433. S2CID 35046348.
External links
[edit]- DEET General Fact Sheet - National Pesticide Information Center
- DEET Technical Fact Sheet – National Pesticide Information Center
- West Nile Virus Resource Guide – National Pesticide Information Center
- Health Advisory: Tick and Insect Repellents, New York State
- US Centers for Disease Control information on DEET
- US Environmental Protection Agency information on DEET
- Review of scientific literature on DEET Archived 2016-12-10 at the Wayback Machine (from a RAND Corporation report on Gulf War illnesses)
- Health Canada - Re-evaluation Decision Document: Personal insect repellents containing DEET (N,N-diethyl-m-toluamide and related compounds), 2002


